Nerves are responsible for how we think, move, feel, and interact with the world—essentially every aspect of our lives—so it is no wonder that the term “nerve damage” can be rather frightening. That fear is often compounded by the common belief that all nerve damage is permanent and irreparable. In today’s blog we are discussing how nerves heal.
As an electrophysiologic specialist I test nerves every day, and I often hear the question, “if there’s nerve damage, then nothing can be done about it, right?”
This idea springs from a long-held belief in neuroscience that humans are born with as many neurons as we will ever have, so any nerve cell that dies is gone forever. Recent discoveries have challenged this notion, with evidence pointing toward some neurogenesis even in adult human brains, but putting aside the birth of new nerve cells, it is a mistake to equate all nerve damage with nerve death.
It turns out that most types of nerve damage will not kill the neurons outright, and most cells that have been damaged can certainly heal so long as the damaging circumstances are treated.
In this article we will discuss the different types and locations of nerve damage which can occur, and the implications each has for a patient’s prognosis.
Cell Bodies vs. Axons
This is the basic anatomy of a neuron:
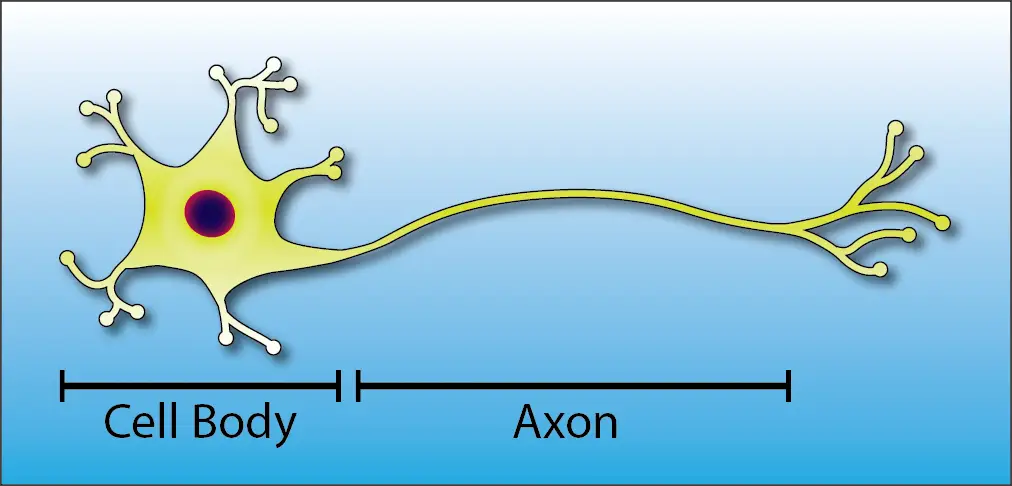
Notice That There are Two Main Structures in a Neuron:
- The cell body is the control center of the neuron. It contains the nucleus (the “brain” of the cell) and the major organelles that are responsible for keeping the cell healthy.
- The axon is the conduit that carries nerve signals throughout the body. At the most, it can be over six feet long, helping our brains to connect with the tips of our toes!
If neurons were lizards, then the cell body would be like the reptile’s head and torso and the axon would be like the tail. As long as the body is healthy the tail can normally be healed.

Luckily our cell bodies are very well protected. The vast majority of them are found within or very close to our skulls and spinal columns, which defend them like a suit of armor.
Conditions which kill the cell bodies directly, including stroke, traumatic brain injury, spinal cord injuries, Parkinson’s and Alzheimer’s diseases, and motor neuron diseases are rare.
Most types of orthopedic nerve injuries, on the other hand, primarily affect axons. This includes radiculopathy (pinched nerves in the back or neck), peripheral neuropathy, carpal/cubital tunnel syndromes, and more.
In each of these cases, if the condition causing the injury is removed, then the cell body will send nutrients down the axon to try and repair the damaged end.
Axon Loss vs. Demyelination
The process of sending nutrients down an axon is known as axoplasmic transport.
If an axon is crushed or severed, then the transport will be disrupted, starving the part of the cell beyond the injury and causing it to degenerate. Like the lizard’s tail, once the cause of injury is removed the axon will gradually regenerate.
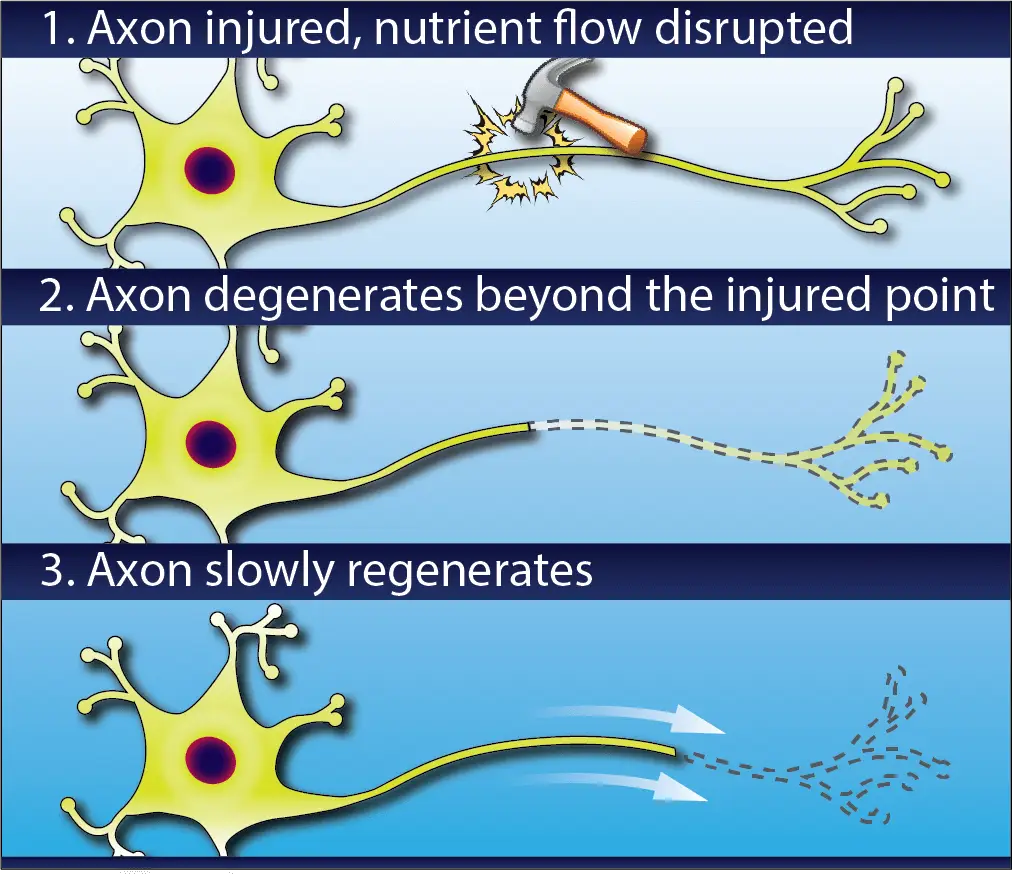
This can take a very long time. Axons re-grow at a rate of 1-3 millimeters per day (about the same rate as your fingernails), so if you’ve ever had a surgery to address an injury at the neck or back, you may have heard your surgeon say that it can take as long as a year to regain strength and sensation.
Luckily, not every nerve injury actually disrupts axoplasmic transport.
Around each axon there are hundreds or thousands of little nodes called Schwann cells, which wrap the axon in a white sheath called myelin which insulates and protects the nerve and helps electrical signals to travel quickly and effectively.
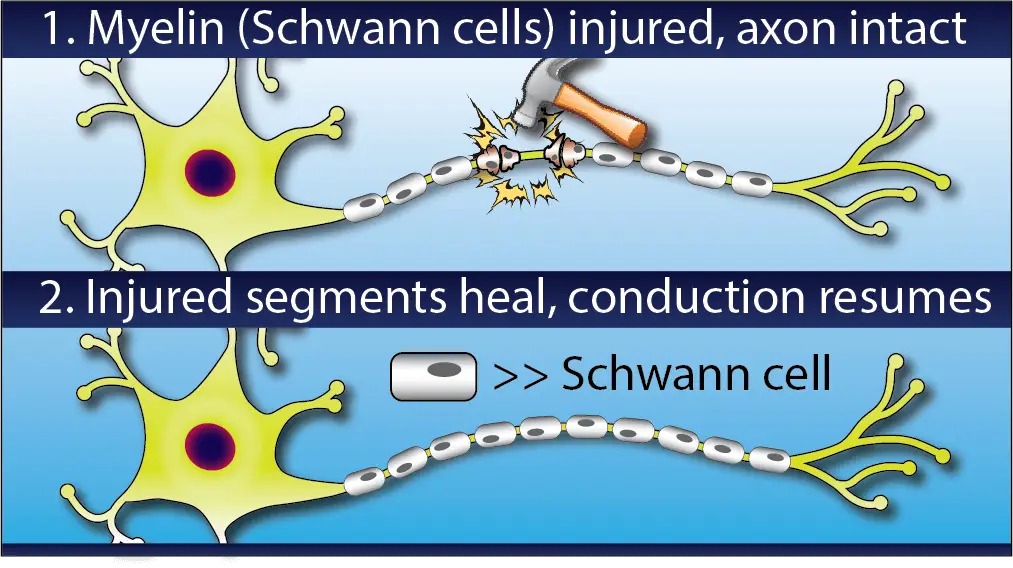
Some nerve injuries only damage this outer myelin sheath. This is called demyelination and will cause symptoms like numbness and weakness because it impairs the conduction of nerve signals, but it has a much better prognosis than actual axon loss.
Demyelinating injuries also tend to heal more quickly than axonal ones. As you can imagine, it takes much less time to re-grow a handful of tiny Schwann cells than to regenerate several feet of axon.
The Endoneurial Tube
If regeneration is required, however, then how do axons know what direction to regenerate to get back to their original targets?
The answer is the endoneurium. This is a layer of connective tissue which runs the length of the axon and forms a thin tube around it. The endoneurium is a fibrous channel, not a cell, so it doesn’t degenerate even when axoplasmic transport is lost and the rest of the axon is starved. When regeneration begins, the axon grows inside the endoneurial tube, which guides it back to its destination.
If the endoneurium is completely severed (i.e. the nerve is cut with a knife, shot with a bullet, etc.), however, then regeneration is far less likely to be successful.
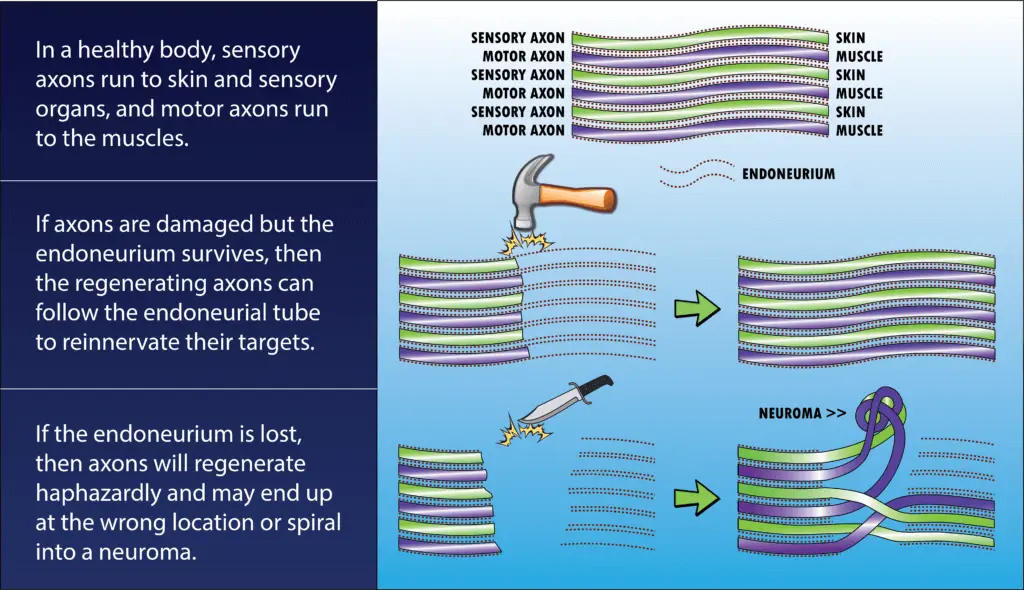
In this case, axons which were meant to go to one muscle might grow into the wrong endoneurial tube and wind up in a different muscle or even the skin. Some axons never manage to find an endoneurium path at all and simply grow in knots and tangles called neuromas.
Moral of the story: try not to get shot or stabbed. Your nerves will thank you.
How Nerves Heal: Regeneration vs. Collateral Sprouting
We’ve discussed a lot about axon regeneration, but you may be wondering what happens to your muscles while they’re waiting around for their neurons to re-grow and get back in touch with them. After all, muscles can’t work without nerves to give them their marching orders.
Unfortunately, if the nerve damage is bad enough, then the denervated muscles may waste away (atrophy). The body is terribly efficient at getting rid of everything it doesn’t think it needs (except for fat, much to my chagrin), and a muscle that hasn’t been contracting may get culled in the months before its axon can reconnect with it.
But most cases are not so bleak. Every nerve contains many, many axons, and if only some of them are damaged then the remaining healthy neurons will come to the rescue.
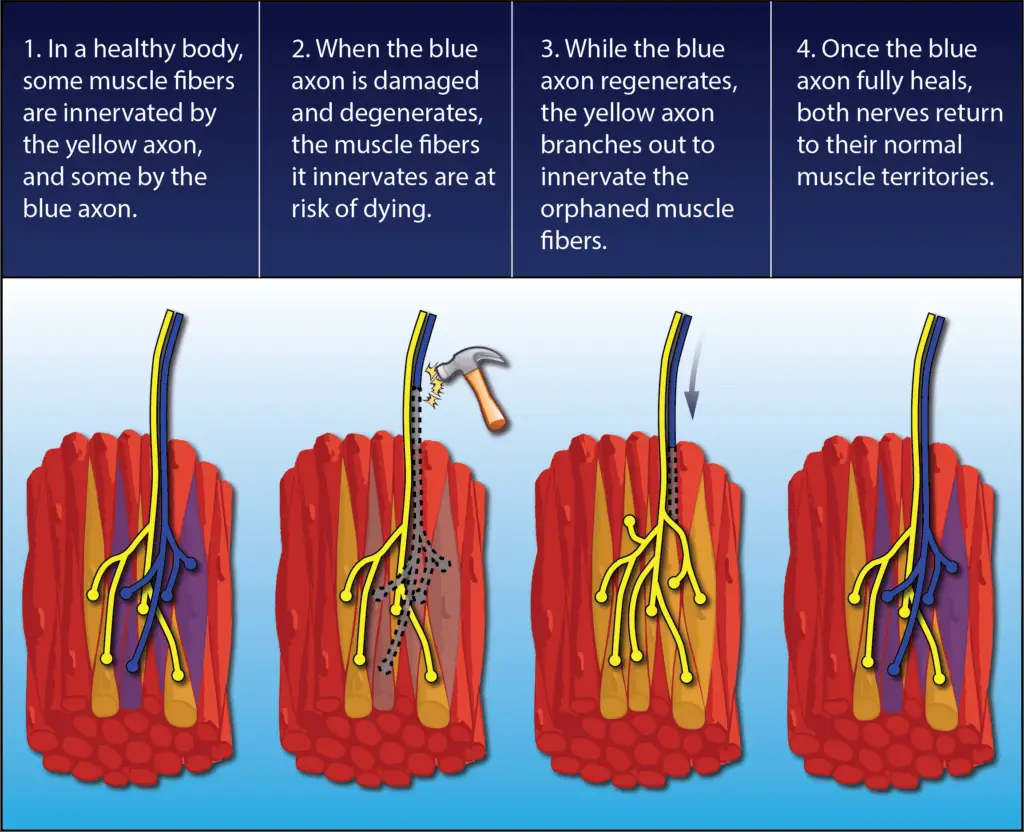
Through a process called collateral sprouting, the ends of the healthy axons will form branches and adopt the orphaned muscle fibers as part of their own responsibility so the muscle can continue to work and remain relevant. This is one reason why after a back or neck surgery you may start regaining strength even before the months required for regeneration have passed.
Just as amazingly, once the damaged axon does find its way back, the fostering nerve returns the extra muscle fibers to their original parent nerve, and the neuromuscular organization reverts to normal.
What Does this Mean for Prognosis?
As you can see, nerve damage is not necessarily permanent. Depending on the details of the injury, better or worse outcomes can be expected.
Here’s a summary of how the principles we’ve discussed will affect prognosis for a given injury:
| BETTER PROGNOSIS | WORSE PROGNOSIS |
| Damage of axons only | Death of cell body |
| Damage of myelin sheath only; axons and axoplasmic transport preserved | Axons and axoplasmic transport disrupted; degeneration occurs and regeneration is required |
| Endoneurial tube preserved | Endoneurial tube disrupted |
| Axon loss incomplete; some axons remain and collateral sprouting occurs | All axons disrupted, muscle reinnervation by regeneration alone |
So how can you find out all these details?
Simple. Electrophysiologic testing.
Through careful evaluation of the function of nerves and muscles, an electrophysiologic specialist can determine if axon loss or demyelination is happening. He or she can also detect if any function is left in a damaged nerve and see signs of collateral sprouting and axon regeneration as they occur.
The simple words “nerve damage” need not strike fear of permanent disability into the heart of every patient. In many cases the miraculous body is capable of healing even injured nerves, and with the right testing, the healing potential of each case can be known.