|
Based on 5.07 monofilament testing there was no statistical difference in lower extremity sensitivity when using monochromatic infrared energy as compared to a placebo. This study is an underpowered, negative trial due to its small sample size and inclusion of the null value. Clinical judgments prove difficult to determine due to sample size. Level of evidence: 2b |
Citation/s:
Clifft, J. K., Kasser, R. J. (2005). “The effect of monochromatic infrared energy on sensation in patients with diabetic peripheral neuropathy.” Diabetes Care 28(12): 2896-2900.
Lead author’s name and fax: Judy Clifft, UTHSC Department of Physical Therapy, 930 Madison Ave., Room 650, Memphis, TN 38163. E-mail: [email protected].
Three-part Clinical Question: For patients with diabetic peripheral neuropathy will monochromatic infrared energy improve sensation?
Search Terms: monochromatic infrared AND diabetic peripheral neuropathy
The Study:
Double-blind, placebo-controlled study without intention-to-treat. Randomization was never stated.
The Study Patients: The subjects consisted of 43 individuals with self-reported diabetes. “Subjects were recruited from the middle Tennessee area and included adult men and women of any race or ethnic background with a confirmed diagnosis of peripheral neuropathy (defined as the inability to detect the 5.07 monofilament at any of the four test sites on the plantar surface of the foot).” Exclusion criteria included those who were pregnant, those with open wounds, or active malignancies. Of the 43 that volunteered only 39 completed the 8-week study. 77 lower extremities with peripheral neuropathy were used. 3 subjects were hospitalized, and one moves out of the area during the study. From the remaining 39 subjects, 35 lower extremities received active monochromatic infrared energy (MIRE) treatment and 35 lower extremities received the placebo MIRE protocol. Semmes-Weinstein monofilaments ranging from 1.65 to 6.65 was used. ““The monofilament was applied to the plantar aspect of the blinded patient’s foot. The test sites were located at the great toe, first metatarsal head, third metatarsal head, and fifth metatarsal head. The sites were randomly tested. Measurements were taken at the beginning of the study (M1), after 4 weeks of MIRE or placebo treatment (M2), and after an additional 4 weeks of non-treatment (M3). The test was scored by the number of sites that could sense the 5.07 or smaller diameter monofilament at M1, M2, and M3.” All measurements were performed by one physical therapist that was blind to group assignment and was not involved in treatment. How participants were assigned to treatment and control groups was not stated.
Control group (N = 35; 35 analyzed): The four MIRE units used in this study were Anodyne Model 120-4 Infrared Therapy Systems. Each device has a main power unit with four flexible therapy pads which contain 50 super-luminous gallium-aluminum arsenide diodes that emit light energy in the near infrared spectrum (890-nm wavelength). Two sham units were used for the treatment of 35 lower extremities in the placebo group. Even though the indicator lights illuminated when the power switch was turned on the units delivered no energy. The therapist who administered the treatment was blinded to which were working units and which were not.
Experimental group (N = 35; 35 analyzed): 35 lower extremities received MIRE treatments. The units were preset by the manufacturer to deliver 1.97 joules · cm -2· min -1when activated. “Each subject sat in a standard chair with socks and shoes removed. The four therapy pads were placed over the following sites in accordance with procedures used by Kochman and recommendations of the manufacturer: 1) distal posterior leg, 2) distal anterior leg, 3) plantar foot over metatarsal heads, and 4) plantar arch of foot. The placement of pads 3 and 4 formed a “T” on the plantar surface of the foot. Commercial plastic wrap was placed between the skin and the MIRE pads for hygienic purposes, and the pads were held in position with neoprene straps supplied by the manufacturer.” “Subjects received the treatment protocol for peripheral neuropathy as recommended by the manufacturer: 30-min treatments three times a week for 4weeks for a total of 12 treatments.”
The Evidence:
|
Outcome |
CER |
EER |
|
Superficial burn |
0 |
5.7% |
|
Measure |
Control Group |
Experimental Group |
Difference |
95% CI |
||
|
Mean |
SD |
Mean |
SD |
|||
|
Sites Sensitive to the 5.07 Monofilament from baseline (M1) to 8wks (M3) |
1.27 |
0.37 |
0.89 |
0.30 |
-0.38 |
-0.95 to 0.19 |
Estimated difference between mean active and placebo measurements: -0.38 (95% CI -0.95 to 0.19)
Comments:
Are the Results Valid?
All volunteers of the study were subject to Semmes-Weinstein monofilament testing by a physical therapist that was blinded to which group the participant belonged. The tester who administered the MIRE treatments was also blinded to which group the participant belonged, as well as the results of the monofilament testing. The study failed to perform any long-term follow-up to determine if there were any side effects. I believe a factor that could have strengthened this study was whether there was a significant difference in the decline of sensitivity since the termination of the study as well as any significant difference in the rate of that decline between placebo and active treatments.
The study never stated whether participants were randomly placed in the active or placebo groups.
The overall strength of this study comes with the active treatment being compared to a placebo. If a placebo group was not used during this study interpretation of the results could have shown to be therapeutically effective. Seeing that both the placebo and active groups improved in sensitivity demonstrates that further follow-up studies also need to be placebo-controlled.
The study did a good job in meeting the power objective. The study sample size was targeted to detect a moderate effect size of f = 0.20 with 80% power given a two-tailed α of 0.05. The power analysis revealed that for an average correlation of subject outcomes over time of r = 0.40, a minimum of 25 subjects was needed in each treatment level to meet the power objective.
The statistical analysis of this study demonstrates that it is a negative trial. It showed that the confidence intervals of the estimated mean between the active and placebo groups included the null value. It also demonstrated that there was no statistical difference between the placebo and active groups. I feel that despite that even if this was not a negative trial it lacked any significant evidence that MIRE increases sensitivity due to the upper boundary of the confidence interval being 0.15 or 15% change of mean between active and placebo.
What Are The Results?
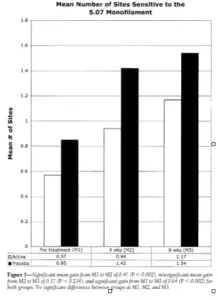
The analysis detected a significant main effect for measurement (P = 0.001) but could detect neither a significant main effect for treatment (P = 0.186) nor for the treatment by measurement point interaction (P = 0.622). The estimated difference between mean active and placebo measurements was negative but non-significant, specifically -0.38 (95% CI -0.95 to 0.19). The Tukey-Kramer procedure was used for follow-up of the significant repeated measure. Estimates, adjusted probabilities, and CIs assessing average change over time were as follows:
- a significant average gain from baseline (M1) to 4 weeks (M2) of 0.47 (P < 0.002; 95% CI 0.16–0.79),
- 2) A non-significant average gain from 4 weeks (M2) to 8 weeks (M3) of 0.17 (P = 0.234;-0.08 to 0.42) resulting in 3) a significant overall average gain from baseline (M1) to 8 weeks (M3) of 0.64 (P < 0.002; 0.22– 1.06) (Fig. 1).
How Can I Apply the Results to Patient Care?
Unfortunately for me, this study has very little clinical value. Due to its threats of validity, it provided no significant evidence for or against the use of MIRE to increase sensitivity in patients with diabetic peripheral neuropathy. Despite this study determining there was no significant difference between MIRE treatments and placebo, I feel further studies need to be completed before coming to a final conclusion. Variation in treatment placement and long-term effects are just two areas that could help strengthen the evidence.